US CDC panel votes to recommend mRNA COVID-19 vaccines over J&J’s | Coronavirus pandemic News
The US had briefly paused use of the COVID jab in April, before finding benefit outweighs very rare, but deadly, risks.
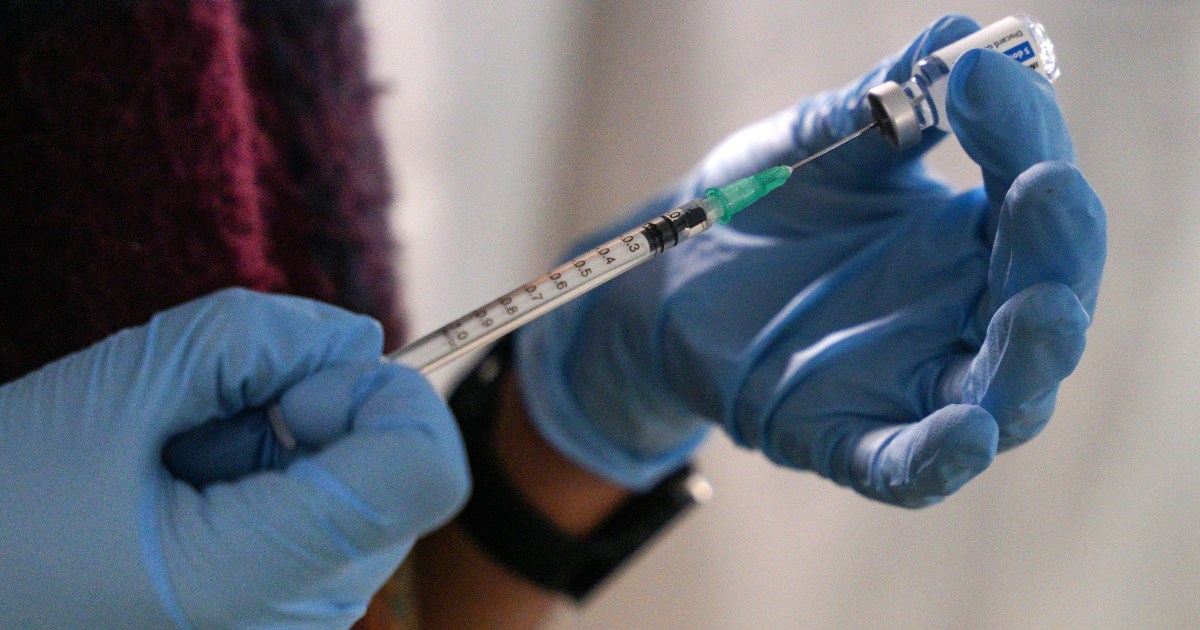
A panel of outside advisers to the US Centers for Disease Control and Prevention (CDC) has voted to recommend Americans choose to receive one of the other two authorised COVID-19 vaccines over Johnson & Johnson’s shot, due to rare but sometimes fatal cases of blood clotting.
The CDC’s Advisory Committee on Immunization voted unanimously on Thursday to make the recommendation. The regulator still needs to sign off on the guidance.
Fewer Americans have received the J&J shot than the other two vaccines by a significant margin. Out of more than 200 million fully vaccinated people in the United States, around 16 million received J&J’s vaccine, according to CDC data.
Cases of thrombosis with thrombocytopenia syndrome (TTS), which involves blood clots accompanied by a low level of platelets, have previously been reported in recipients of the J&J vaccine. The highest reporting rates are in women under 50.
The CDC said that the rate of such incidents is higher than previously estimated, both in women and men. The agency has identified more than 50 cases of TTS in the US, about 3.83 cases per million J&J doses administered.
At least nine people have died following the blood clotting incidents in the US, the CDC has said.
Members of the panel also said J&J’s vaccine is less effective in preventing COVID-19 than the other two authorised vaccines.
In a presentation to the committee, a leading J&J vaccine scientist said the vaccine generates a strong and long-lasting immune response with just a single shot.
“In the setting where many people do not return for a second dose or a booster, the durability of the single shot Johnson & Johnson vaccine as a primary regimen could make a crucial difference in saving lives in the US and around the globe,” J&J’s Dr Penny Heaton said.
In the US, the shot has been useful for reaching some hard-to-reach populations and settings like the homeless and residents of corrections facilities.
J&J’s vaccine uses a technology based on a modified version of an adenovirus to spur immunity in recipients, while the other two authorised vaccines use messenger RNA technology.
J&J’s one-dose vaccine received emergency use authorisation in late February. In April, US regulators paused administering the vaccine for 10 days in order to investigate the blood clotting.
A CDC scientist said on Thursday that the rate of deaths from TTS did not decrease after the pause in April.
J&J shares closed up around 1 percent on the New York Stock Exchange. The company sells the vaccine at a not-for-profit price, so it has not been a significant revenue driver.